THE DRUG DEVELOPMENT PROCESS
The complexity of drug development has greatly increased over the past 40 years. The process requires a preclinical phase of drug development, an investigational new drug (IND) application, and complete clinical testing before marketing approval from the FDA. Generally, new drug applications (NDAs) or biologics license applications (BLA) are carefully reviewed before approval, and then drug performance is resubmitted to regulatory agencies for post-marketing studies. The overall goal of this process is to bring safer, more efficient treatments to patients as quickly as possible after a thorough medical evaluation.
There are five critical steps to the drug development process in the United States. These steps are outlined in the diagram and text below.
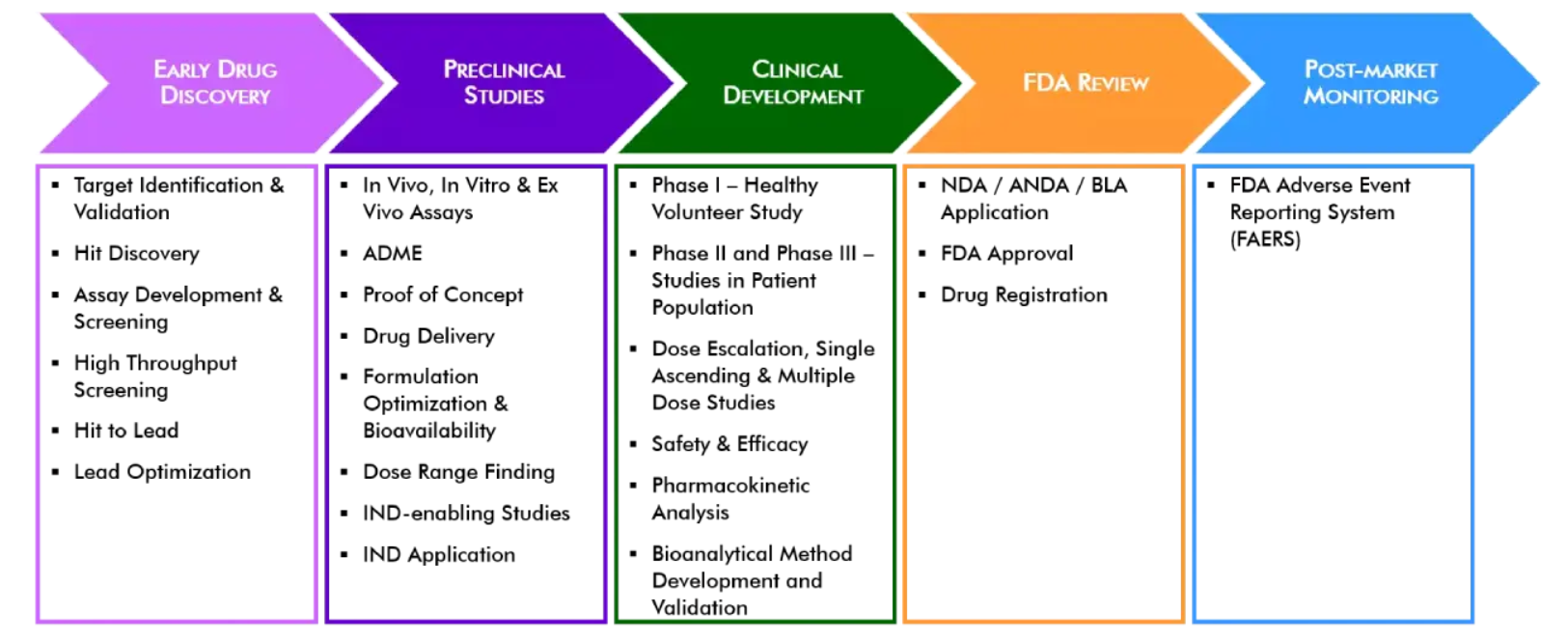
Step 1: Discovery and Development – Research for a new drug begins in the laboratory.
Typically, researchers discover new drugs through:
-
-
- New insights into a disease process that allow researchers to design a product to stop or reverse the effects of the disease.
- Many tests of molecular compounds to find possible beneficial effects against any of a large number of diseases.
- Existing treatments that have unanticipated effects.
- New technologies, such as those that provide new ways to target medical products to specific sites within the body or to manipulate genetic material.
-
At this stage in the process, thousands of compounds may be potential candidates for development as a potential medical treatment. After early testing, however, only a small number of compounds look promising and call for further study.
Once researchers identify a promising compound for development, they conduct experiments to gather information on:
-
-
- How it is absorbed, distributed, metabolized, and excreted.
- Its potential benefits and mechanisms of action.
- The best dosage.
- The best way to give the drug (such as by mouth or injection).
- Side effects or adverse events that can often be referred to as toxicity.
- How it affects different groups of people (such as by gender, race, or ethnicity) differently.
- How it interacts with other drugs and treatments.
- Its effectiveness as compared with similar drugs.
-
Step 2: Preclinical Research – Drugs undergo laboratory and animal testing to answer basic questions about safety.
Before testing a drug in people, researchers must find out whether it has the potential to cause serious harm, also called toxicity. The two types of preclinical research are in vitro and in vivo.
FDA requires researchers to use good laboratory practices (GLP), defined in medical product development regulations, for preclinical laboratory studies. The GLP regulations are found in 21 CFR Part 58.1 (Good Laboratory Practice for Nonclinical Laboratory Studies). These regulations set the minimum basic requirements for study conduct, personnel, facilities, equipment, written protocols, operating procedures, study reports, and a system of quality assurance oversight to help assure product safety.
Usually, preclinical studies are not very large. However, these studies must provide detailed information on dosing and toxicity levels. After preclinical testing, researchers review their findings and decide whether the drug should be tested in people.
Step 3: Clinical Research – Drugs are tested on people to make sure they are safe and effective.
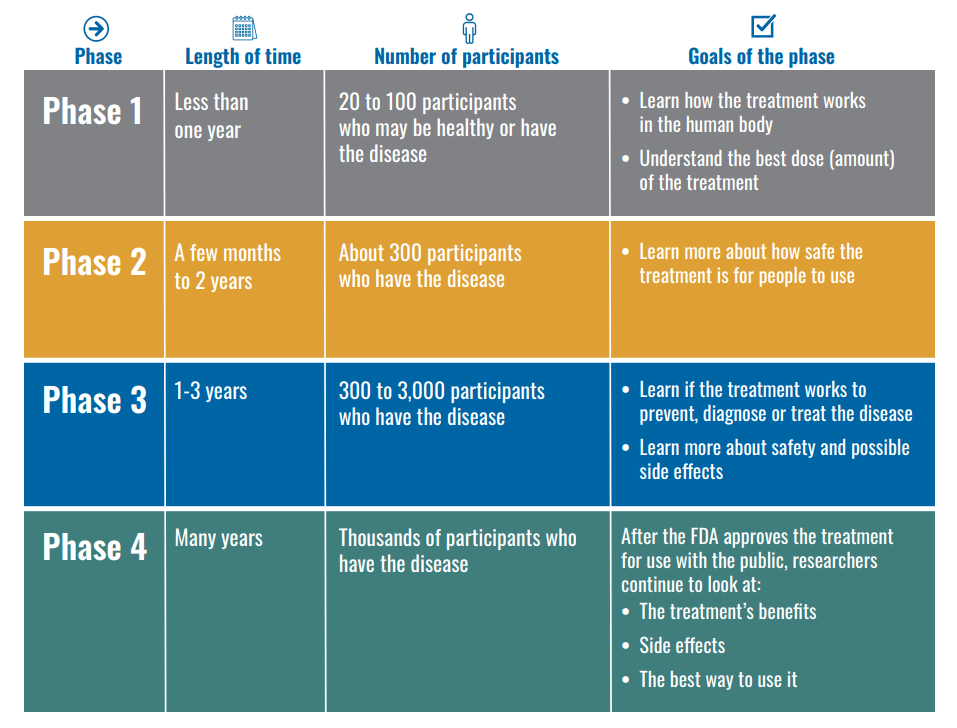
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins. Drug developers are free to ask for help from FDA at any point in the drug development process.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports. This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
Step 4: FDA Review – Review teams thoroughly examine all of the submitted data related to the drug or device and make a decision to approve or not to approve it.
If a drug developer has evidence from its early tests and preclinical and clinical research that a drug is safe and effective for its intended use, the company can file an application to market the drug. The FDA review team thoroughly examines all submitted data on the drug and makes a decision to approve or not to approve it.
A New Drug Application (NDA) tells the full story of a drug. Its purpose is to demonstrate that a drug is safe and effective for its intended use in the population studied. A drug developer must include everything about a drug—from preclinical data to Phase 3 trial data—in an NDA. Developers must include reports on all studies, data, and analyses. Along with clinical results, developers must include proposed labeling, safety updates, drug abuse information, patent information, any data from studies conducted outside the U.S., IRB-compliance information, and directions for use.
Once FDA receives an NDA, the review team decides if it is complete. If it is not complete, the review team can refuse to file the NDA. If it is complete, the review team has 6 to 10 months to make a decision on whether to approve the drug.
In cases where FDA determines that a drug has been shown to be safe and effective for its intended use, it is then necessary to work with the applicant to develop and refine prescribing information. This is referred to as “labeling.” Labeling accurately and objectively describes the basis for approval and how best to use the drug.
Often, though, remaining issues need to be resolved before the drug can be approved for marketing. Sometimes FDA requires the developer to address questions based on existing data. In other cases, FDA requires additional studies. At this point, the developer can decide whether or not to continue further development. If a developer disagrees with an FDA decision, there are mechanisms for formal appeal.
Step 5: FDA Post-Market Safety monitoring – FDA monitors all drug and device safety once products are available for use by the public.
Even though clinical trials provide important information on a drug’s efficacy and safety, it is impossible to have complete information about the safety of a drug at the time of approval. Despite the rigorous steps in the process of drug development, limitations exist. Therefore, the true picture of a product’s safety actually evolves over the months and even years that make up a product’s lifetime in the marketplace. FDA reviews reports of problems with prescription and over-the-counter drugs, and can decide to add cautions to the dosage or usage information, as well as other measures for more serious issues.
