CGMP IN A NUTSHELL
- Know what you are supposed to do before you do it.
- Document everything that occurs.
-
- According to the FDA, “if it isn’t documented, it didn’t happen.”
- Ensure consistency and control over all processes
- Have an independent group make the final decision on a product to avoid bias.
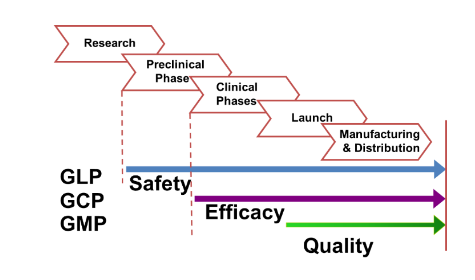
GLP = Good Laboratory Practices
GCP = Good Clinical Practices
GMP = Good Manufacturing Practices
