The guiding source for Biotility’s BACE Exam administration policies and procedures is the Standards for Educational and Psychological Testing, which focuses on ensuring each candidate is tested fairly and professionally and on giving all candidates an equal opportunity to demonstrate their knowledge. To ensure Biotility’s exam administration standards are upheld, all personnel who participate in the administration of the BACE must be trained and certified through the BACE Administrator Certification Process.
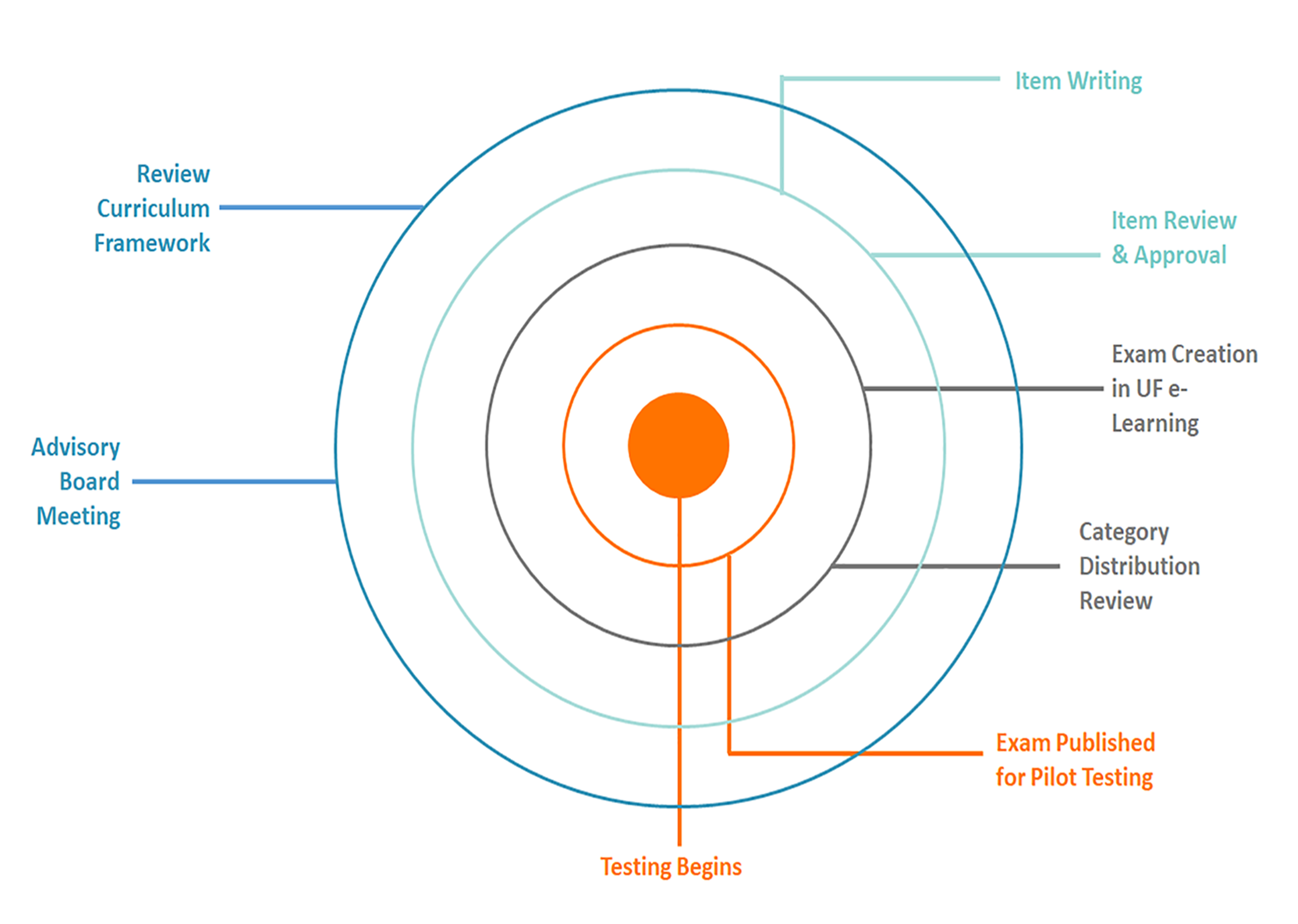
Exam questions are written and validated by subject-matter experts in the industry, and the complete exam is vetted and approved annually by Biotility’s national Advisory Board to confirm:
- The scientific accuracy of individual questions
- The alignment of each question with its corresponding industry-defined blueprint standard
- The overall relevance of the exam to industry requirements for technician-level positions
The credentialing standards for BACE include the Academic Knowledge and CTE Performance Standards and Benchmarks taught in Industrial Biotechnology programs. Certified individuals have a knowledge and skill set applicable to entry level positions in the biotechnology industry (Standards may be found here: 2019-2020 Industrial Biotechnology Academic Knowledge and CTE Performance Standards). These standards are reviewed annually by our national Advisory Board.
National Advisory Board
| Akron Biotech | Manager, Strategic Alliances | Florida |
| Anemoi Biotech Holdings, LLC | Principal Scientist | Iowa |
| AxoGen, Inc. | Product Development Engineer | Florida |
| ELISA Technologies | Scientific Director | Florida |
| Evergreen Bioscience Innovation | Chief Executive Officer | Washington |
| Exogenesis Bio Inc., Gene Therapy CMC | Co-Founder and CTO | California |
| Georgia Bio | VP Operations | Georgia |
| Integra Life Sciences | Senior Product Manager | Maryland |
| Labcorp Drug Development | VP, Early Development | North Carolina |
| Merck & Co. | Associate Director, Engineering | Pennsylvania |
| Novartis Gene Therapies | Senior Scientist, Analytical Science & Technology | North Carolina |
| QuickStart | Director, Georgia BioScience Training Center | Georgia |
| Regeneron | Research and Development Specialist | New York |
| Yukon Medical, LLC | Senior Director, Product Development | North Carolina |

Advisory Board Letter of Support Letter-of-Support-2024BACE
