Institutional Review Board (IRB) Specialist Credential
Credential Overview
Ethical oversight and regulatory compliance are cornerstones of human subjects research. The IRB Specialist Credential (IRBSC) validates the knowledge and skills required to support Institutional Review Board operations and uphold ethical standards in diverse research environments. This credential demonstrates preparedness to assist in the review of protocols, evaluate informed consent practices, manage compliance processes, and ensure that research aligns with federal regulations and institutional policies.
Credential Highlights
- Demonstrates understanding of federal and international research regulations, including 45 CFR 46, 21 CFR Parts 50 and 56, and ICH-GCP
- Verifies ethical and procedural knowledge in informed consent, risk/benefit assessment, and protection of vulnerable populations
- Confirms competence in compliance monitoring, documentation practices, and IRB decision-making processes
- Signals readiness for roles in IRB offices, research administration, compliance oversight, and human research protections
Whether launching a career or deepening expertise in research compliance, the IRBSC provides a recognized foundation for success.
Need Help or Have Questions?
Biotility is available to answer any questions about the APTCE registration process.
Email: biotilitycs@research.ufl.edu
Phone: 386.462.3181 Option #1
Earning Your Credential: The IRBSCE Exam
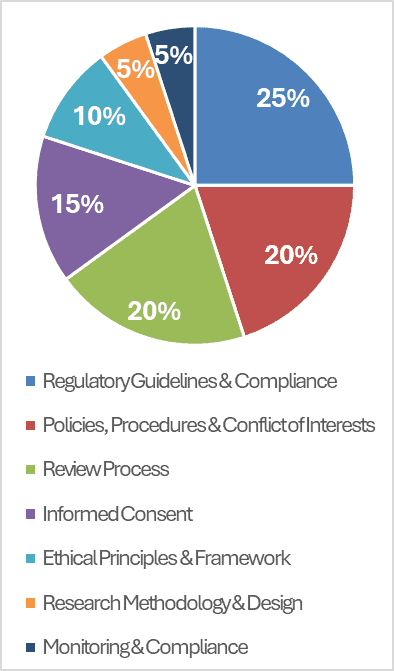
To earn the IRB Specialist Credential, candidates must pass the IRB Specialist Credentialing Exam (IRBSCE). This industry-aligned exam evaluates knowledge across seven critical domains: ethical principles, informed consent, monitoring and compliance, conflict of interest and institutional policy, regulatory frameworks, research methodology, and IRB review processes.
The IRBSCE is a closed-book, computer-based exam delivered through the University of Florida’s e-Learning platform and remotely proctored via ProctorU. The exam consists of 75 multiple-choice, true/false, and scenario-based questions, administered in a single two-hour session. A passing score of 80 percent is required to earn the credential, which is valid for five years.
Exam Fee: The IRBSCE costs $215 per attempt.
Aligned with ISO guidelines and designed for real world application, Biotility’s credentialing exams are created in collaboration with biotechnology employers and overseen by a national industry advisory board.
